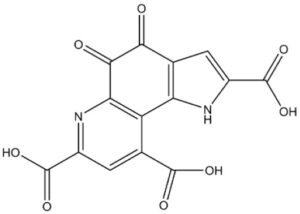
August 5, 2023- The US Food and Drug Administration (FDA) approved Zurzuvae (Zuranolone), which is the first oral medication used to treat adult postpartum depression (PPD). Previously, in clinical practice, psychological intervention was mainly used or intravenous injections could only be administered by medical staff in some medical institutions, lacking drugs tailored to the safety needs of pregnant women.
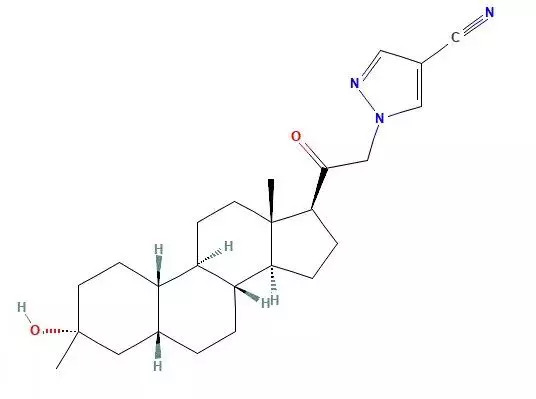
Postpartum depression (PPD) refers to the occurrence of obvious depressive symptoms or typical episodes of depression in women during the postpartum period. Along with postpartum restlessness and postpartum psychosis, it belongs to the category of postpartum mental syndrome, mainly due to rapid changes in hormone levels in the body after childbirth.
Like other forms of depression, PPD is characterized by sadness and/or loss of interest in past activities, as well as a decrease in the ability to experience happiness. It may experience symptoms such as cognitive impairment, sadness or inadequacy, loss of energy, or suicidal ideation, with an international prevalence rate of over 10%.
The clinical manifestations of PPD include: low mood, loss of interest in activities, changes in sleep patterns and appetite, decreased energy, feelings of guilt or lack of value, lack of concentration, and even a tendency to harm infants or commit suicide.
The pathogenesis of PPD is not yet clear and can be broadly divided into two major factors: physiological and psychological. Physiological factors are a significant decrease in hormones in the body of pregnant women after childbirth; Psychological factors are mainly influenced by various factors such as the environment.
Postpartum depression is a serious and potentially life-threatening disease that can cause women to feel sadness, guilt, lack of self-worth, and even in severe cases, develop thoughts of harming themselves or their children. Moreover, postpartum depression can disrupt maternal infant relationships and have an impact on children’s physical and emotional development. “Tiffany Farchione, director of the Department of Psychiatry at the FDA Center for Drug Evaluation and Research For many women, obtaining oral antidepressants will be a beneficial option to cope with extreme and sometimes life-threatening emotions.

ZURZUVAE is the first and currently the only oral PPD drug. As a quick acting drug, the recommended daily dosage for ZURZUVAE is 50 milligrams. Once a day for 14 days can quickly improve the depressive symptoms of women with PPD, but it is not suitable for long-term use.
On the other hand, it requires understanding, attention, and support from loved ones and family members. Prevention outweighs treatment, and treatment can only serve as the ultimate measure, providing pregnant women with more attention and listening, companionship, and childbirth; Pay attention to patient education and seek the help of professional doctors when necessary. Perhaps emotional support is the “best cure” for PPD.